What is Serialization?
Counterfeit drugs are a constant threat for public health worldwide. They are dangerous as they contain unknown substances and side effects. To combat these fake drugs, the governments have developed a counter method. The goal? Improve traceability of drugs produced in pharmaceutical companies. A first step to accomplish this goal is to implement serialization. Implementing a unique serial number to each prescription drug unit will help to track the drug from the manufacturing stage to the distribution stages.
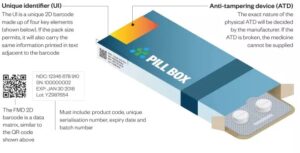
All known serialization systems are based on the use of this serial number, hence the name. The serial number is added to the other already legally enforced data such as batch number, expiration date, and product origin. The products also receive a Global Trade Item Number (GTIN), it is the combination of the Serial Number and the GTIN that leads to a Unique Identifier of the product (UID). The GTIN also contains a manufacturer identifier –called a Company Prefix- and an index number that is used to define the packaging level (bottle, carton, bundle, case or pallet) which comes in handy when doing aggregation.
The Process
This means that all packaging levels receive a serial number through a parent-child relationship. E.g., one pallet serial number points at 10 case serial numbers, each case serial number points at 100 carton serial numbers inside each case. With one single scan of the pallet serial number one will know all 1000 carton serial numbers on that pallet in one go. It’s no secret that distributors, wholesalers and other stakeholders prefer this to just serialization. With “simple” serialization, and no aggregation, all the cases would need to be opened one by one and cartons scanned one by one to identify them individually. Aggregation would make operations easier, more efficient and safer when it comes to product recalls.
MAH’s
Serial numbers are often provided by the marketing authorization holders (MAH’s) IT platform or by 3rd party companies, called Level 4 systems. Level 4 systems are very often cloud based services. The numbers can also be generated on premise or even on the line itself. The 3rd party services require a monthly or yearly fee to deal with all data related matters such as serial number generation, reconciliation report processing and interface with the government repositories such as the National Medicines Verification System (NMVS) and the European Medicines Verification system (EMVS). The Level 4 systems can play an important role in connecting all the stakeholders. This is a big difference between the US and the EU. The EU is similar to the Turkish system where the authorities have set up a central data repository and where the stakeholders can connect to. The US on the contrary do not have such a repository, the data stays at the manufacturer and is commonly made available to the stakeholders by means of 3rd party platforms. The wholesalers will be some kind of intermediate level connecting to the points of dispense and the manufacturers.
Track and Trace
The Turkish system is the only real end-to-end Track & Trace (T&T) system in the world. In Turkey all the stakeholders must connect to the central Turkish repository and all data changes at distributors, wholesalers, and such must be updated in that system. An example would be that of a distributor unpacking a pallet with 100 cases and building two new pallets with 50 cases each. It would require two new pallet labels with new serial numbers, those serial numbers will point at 50 cases each and the two serial numbers will go into the repository while the old pallet number is destroyed. That activity is recorded in the Turkish authority database together with the status and the necessary time stamps, full T&T it is called.
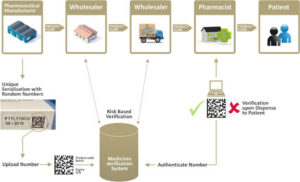
figure 1 Point of dispense medicines verification
The Data
In the EU some of the stakeholders can connect to the central repository for verification purposes only, they cannot alter the data in that system, they are free to make 100 pallets with one case on each pallet, it will not show up in the central repository. But, a good 3rd party platform (Level 4) could offer a service to connect the MAH, distributors and wholesalers and log those changes. It becomes practically a full T&T after all. The 3rd party service leads to a “beyond compliance benefit”. The part of the compliant data residing in the central repository will not change since there is no aggregation data inside that system, only serialization data!
An MAH will provide their contract manufacturing organizations (CMO’s) with serial numbers over such 3rd party platforms or over their own IT platform. Some even work over a portal system delivering data over the air that is later manually brought to the line by means of a USB stick. For smaller companies without large IT resources a 3rd party platform is the best way to go.
Interested in learning more? Contact us today to find out how we can help with your technical needs.
Similar posts


