Introduction
CAR-T therapy is a groundbreaking form of cancer treatment that harnesses the power of the immune system to fight disease. This innovative approach involves genetically modifying a patient’s T-cells to recognise and destroy cancer cells. However, the success of CAR-T therapy depends on a sophisticated manufacturing process. SPGL supports its clients in advancing this innovation by providing expertise in the design, construction, commissioning, qualification and validation of these facilities. This article examines the key steps involved in producing CAR-T cells and their impact on cancer treatment.
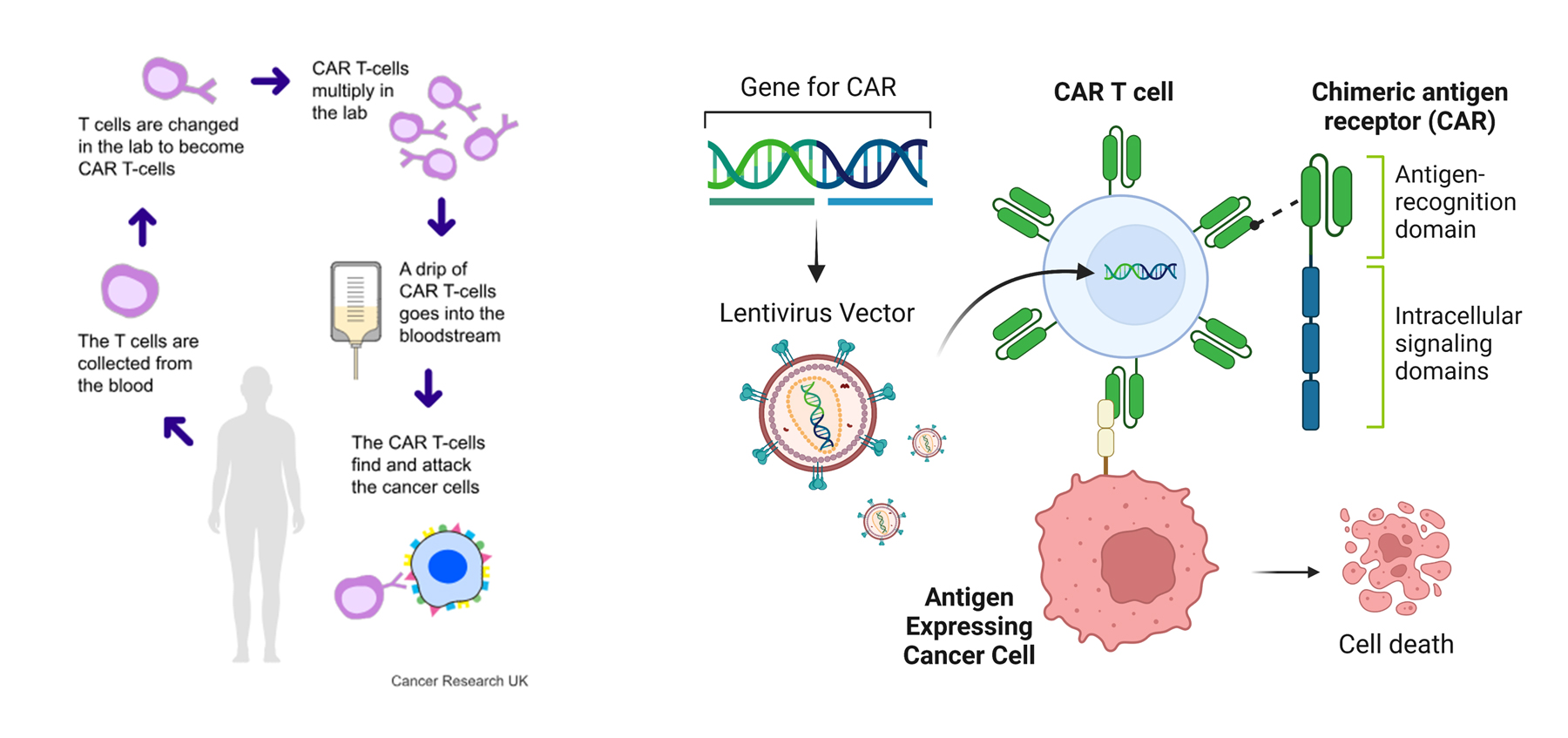
What is CAR-T Therapy
Chimeric Antigen Receptor T-cell (CAR-T) therapy is a revolutionary approach in the field of cancer immunotherapy, offering hope to patients with certain types of cancers that are resistant to conventional treatments. CAR-T involves engineering a patient’s own T-cells to recognise and attack cancer cells, making it a form of individualised therapy with incredible potential. However, the success of this therapy is closely tied to a highly complex and sophisticated manufacturing process.
The CAR-T Manufacturing Process
1. Patient Leukapheresis
The process begins with “leukapheresis”, a procedure that collects white blood cells, particularly T-cells, from the patient. These cells are separated from other blood components, such as red blood cells and plasma, and their quality is critical to the therapy’s success.
2. T-Cell Activation and Transduction
Once collected, the T-cells are activated and genetically modified. In this stage, the cells are stimulated to multiply and are transduced using a viral vector (typically a lentivirus or retrovirus) to introduce the gene encoding the chimeric antigen receptor (CAR). This enables the engineered T-cells to recognise specific antigens on cancer cells.
3. Cell Expansion
The modified T-cells then undergo “expansion”, to grow them to a sufficient number in a controlled environment. This occurs in small bioreactors (such as wave bags or flasks), which provide the necessary growth conditions to ensure that enough CAR-T cells are produced to fight the specific cancer effectively.
4. Quality Control and Testing
Throughout the process, stringent quality control measures are in place to ensure the safety, potency, and purity of the CAR-T cells. Tests confirm that the CAR-T cells express the correct CAR, are contamination-free, and function as intended. All tests have to pass prior to the CAR-T cells being released for clinical use.
5. Cryopreservation and Transport
After testing, the CAR-T cells are cryopreserved (frozen) to maintain their viability and transported to the healthcare facility where the patient will receive treatment.
6. Infusion into the Patient
The final step is “infusion”, where the engineered CAR-T cells are delivered back into the patient’s bloodstream. Following preparative chemotherapy to reduce the patient’s existing immune cells, the CAR-T cells are delivered into the patient’s bloodstream. Once inside, they seek out and destroy cancer cells expressing the target antigen. CAR-T therapy has shown remarkable efficacy, especially in treating certain types of blood cancers, such as leukemia and lymphoma.
Summary
CAR-T therapy represents a breakthrough in individual therapy, offering new hope to cancer patients and transforming the future of oncology. Although the manufacturing process is complex, continuous advancements in production techniques are expected to improve the scalability and accessibility of CAR-T therapies, bringing this life-saving treatment to more patients around the world.
List of References
Interested in learning more? Contact us today to find out how we can help with your technical needs.
Similar posts



